Adopting inclusive pharmaceutical packaging requires paying more attention to design and developing packaging, labels, and cases easily accessible to people with disabilities or impairments, to improve the buying experience for all consumers. Today, there are expedients regulated by law or strongly recommended by professionals to ensure maximum intuitiveness in recognition during the purchase and use of pharmaceutical products.
Accessible packaging: an issue not to be underestimated
In the pharmaceutical sector, is essential making the information clear and simple for everyone to distinguish the packaging on the shelves, read the information shown, and give access to the product inside. This is why the issue of inclusive packaging, accessible even to the most disadvantaged groups among consumers, is gaining importance.
The emergence of initiatives such as The Ethical Packaging Charter, published in 2015, further highlights the necessity of overcoming those obstacles that make it difficult for disadvantaged people to read and use certain products. This publication refers precisely to the necessity of packaging to be intuitive and to communicate effectively to everyone.
If the packaging has to meet the needs of users, several solutions can be found in the design phase to facilitate reading and interactions with packages. Let’s look at some of them.
Braille embossing on a cardboard box
This solution is especially useful for visually impaired or blind people to facilitate comprehension of the product type they are about to purchase. In the pharmaceutical sector, the obligation to include the drug’s commercial name and dosage in Braille applied to the outer carton packaging is regulated by Law No. 149 of July 26, 2005. Braille embossing is carried out through the process of die-cutting or gluing, by which embossing dots are created directly on the outer carton, to enable tactile perception and embossed reading of the text on cardboard.
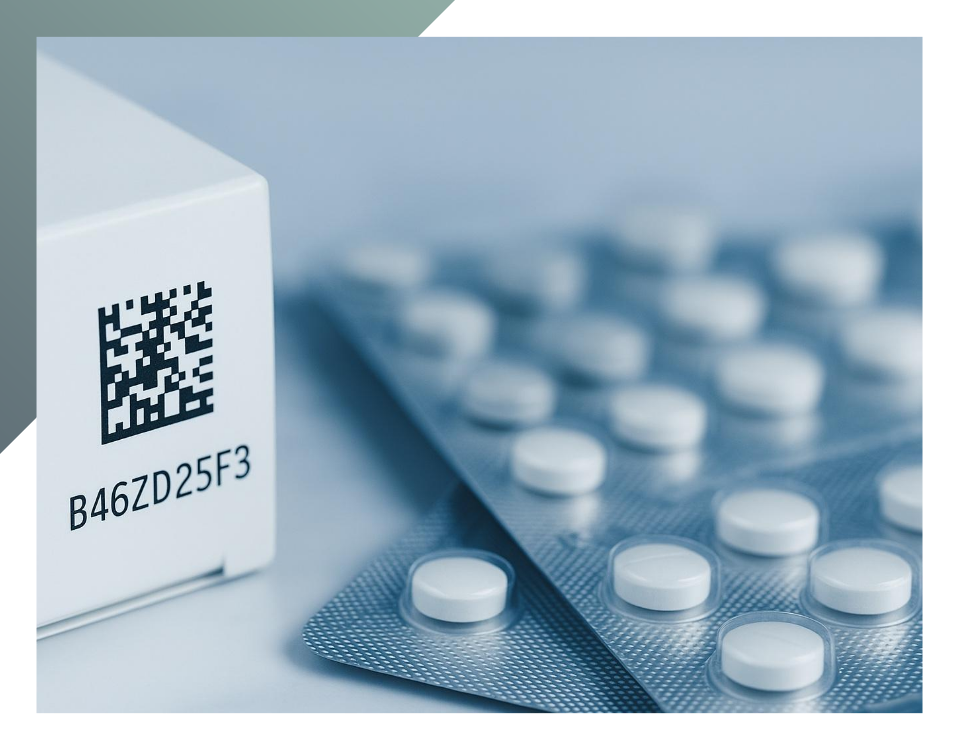
QR codes
Printing QR codes on the outer carton of a drug is a useful solution to make it even easier to access product information. The QR code provides a link that can be accessed to obtain a digital package insert with the same information as in the printed insert, which is always present in the package anyway. The advantage of the QR code is that it makes patient information available in multiple languages: contraindications, mode of use, dosage and other useful indications.
Easy openings and contrasting graphics
Visually impaired, people with movement problems or neurodiversity can find it difficult to both recognize a product on the shelf and open it once purchased. More and more companies today invest in designing easy openings that do not involve, for example, forcing sealed caps, the use of plastic bands or a second tool, but employ light and flexible materials, with easy gripping points and intuitive dispensing systems.
Packaging graphics also play an important role in product communication, not just in terms of marketing. Unlike OTC drugs, Legislative Decree No. 219 of April 24, 2006 (through which Directive 2001/83/EC was transposed in Italy and, later amended by Legislative Decree 274/2007) sets some specific stringent canons on the use of signs and fonts for the graphics of the texts shown on the packaging of pharmaceutical products sold by prescription. These design rules are intended to facilitate the identification of the product in relation concerning product categories and other medicines on sale.
For non-pharmaceutical products (nutraceuticals, dietary supplements) or OTC drugs, size and clear letters represent a solution that aims at inclusivity and more effective communication: contrasts between different colors, more manipulable shapes, clearer fonts, and a precise spatial organization of the information visible on the packaging.
With Eurpack, custom packaging also becomes inclusive and easily accessible. The Packaging Development Center shares every stage of 3D design and development with customers to identify the best solutions to the manufacturing needs of the pharmaceutical industry.
Come possiamo aiutarti?
Se desideri approfondire il nostro impegno verso la sostenibilità o hai domande sui nostri progetti e sulle nostre iniziative, siamo a tua disposizione per offrirti tutte le informazioni di cui hai bisogno.